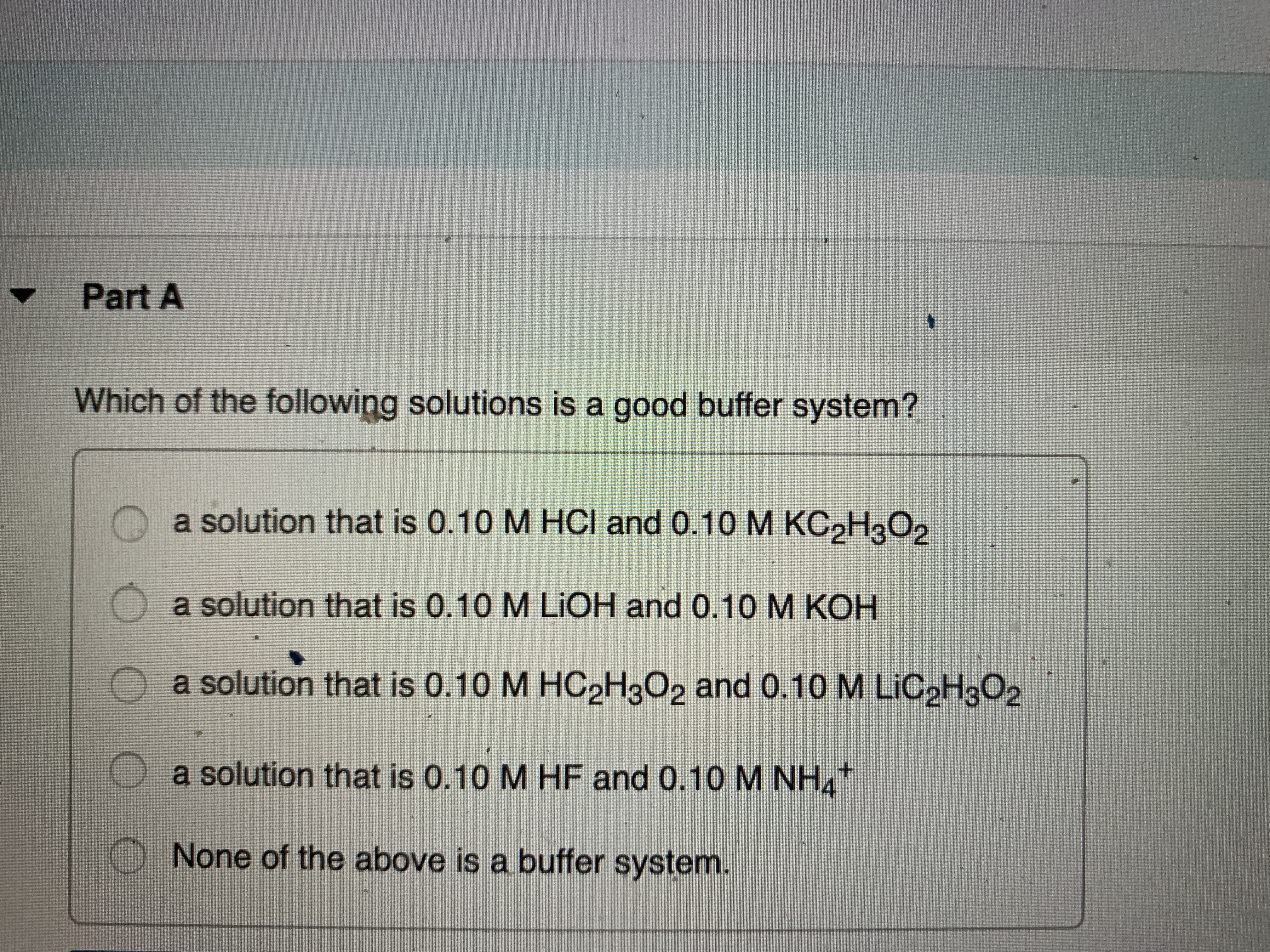
Which Of The Following Is A Buffer System
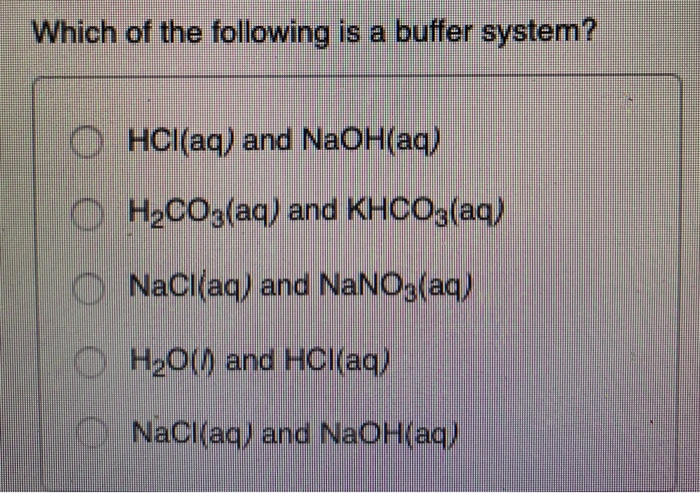
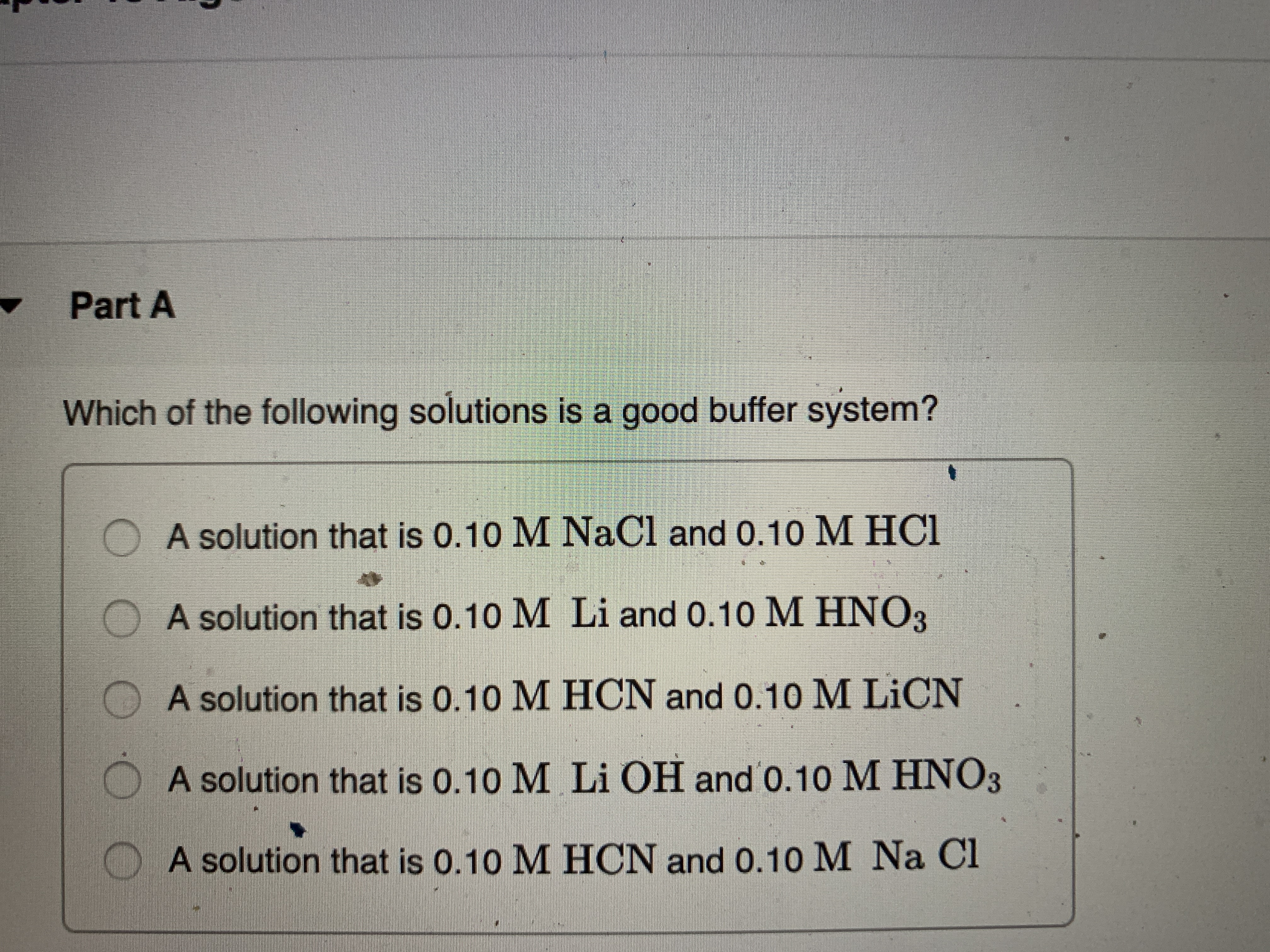
Which of the following is a buffer system. 1 H_3PO_4 is a weak acid and its conjugate base H_2PO_4- is a weak base. A a solution that is 010 M NaOH and 010 M HNO3 B a solution that is 010 M NaCl and 010 M HCl C a solution that is 010 M HCN and 010 M NaCl D a solution that is 010 M HNO3 and 010 M. It can be made either in a weak acid and salt or can be a weak base and its salt.
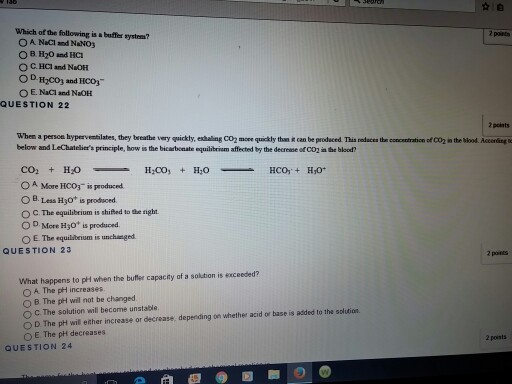
Sending packets that when reassembled are too large for the system to understand B. At the time when the acid is added to it. Strong acid HCl buffered by weak base NaHCO3 One way the kidneys maintain HCO3- balance is by __________.
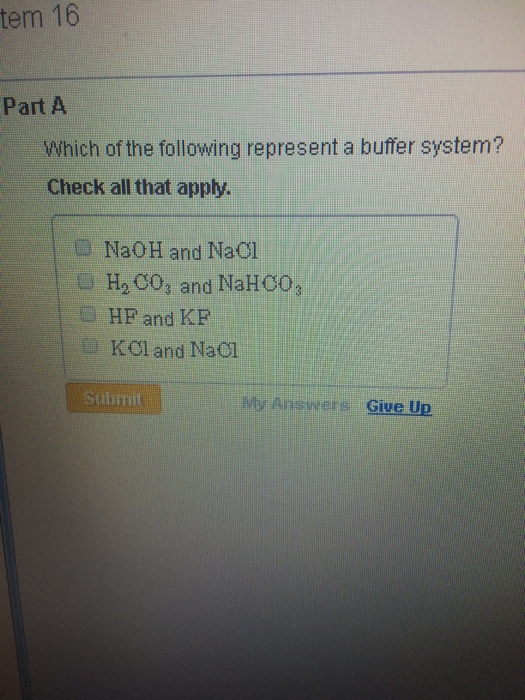
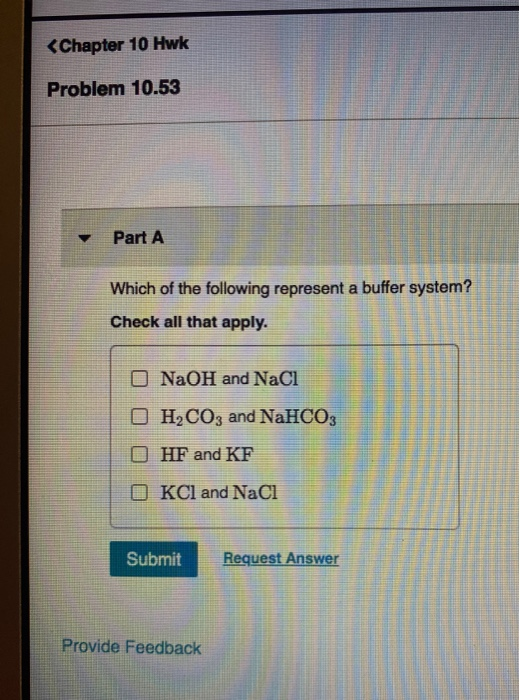
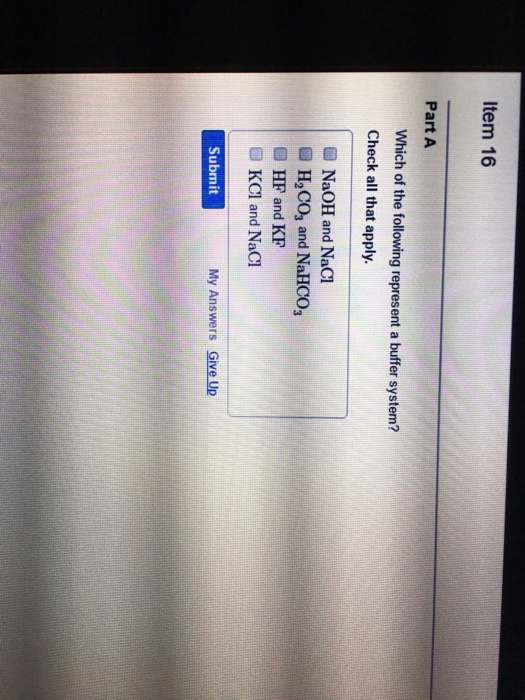
Buffer solution is the weak acid and its conjugate base or weak base. NaOH and NaCl HC2H3O2 and NaC2H3O2 H2S and KHS KCl and NaCl. Which of the following is a buffer system.
Which of the following is NOT a buffer system of the body. Buffer overflow is a software coding error that enables hackers to exploit vulnerabilities steal data and gain unauthorized access to corporate systems. Sending packets very quickly to fill up the receiving buffer D.
The pH of the solution is maintained through an acid-base reaction wherein added. Sending TCP packet with the fragment offset out of bounds Answer. A solution that is 010 M HCN and 010 M NaCl.
The HCN is a weak acid but its conjugate base is CN that is not present in that solution. IS NOT A BUFFER. After carbon dioxide is dissolved it combines with the water.
Heres my question. Chemistry questions and answers.
Previous question Next question.
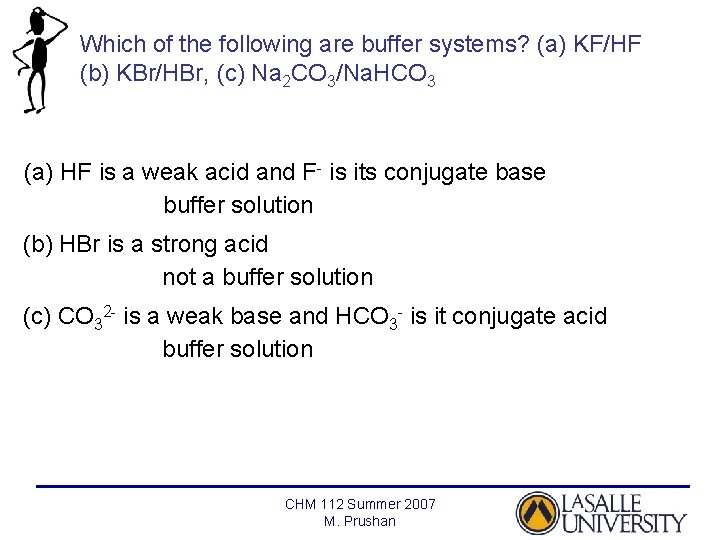
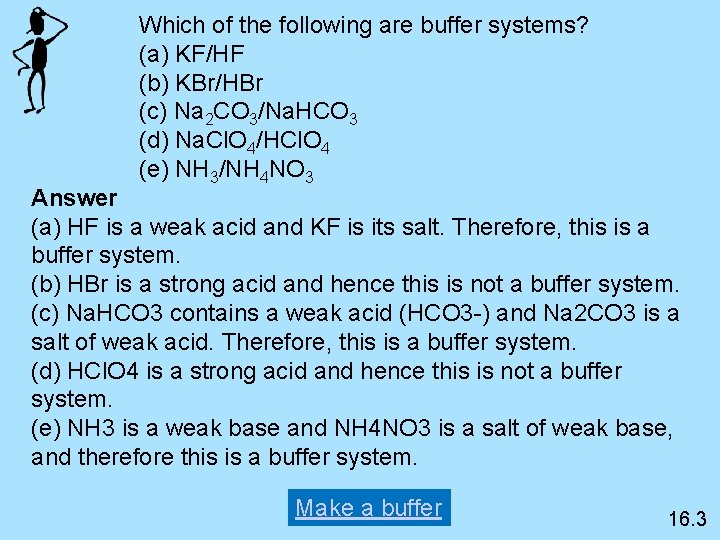
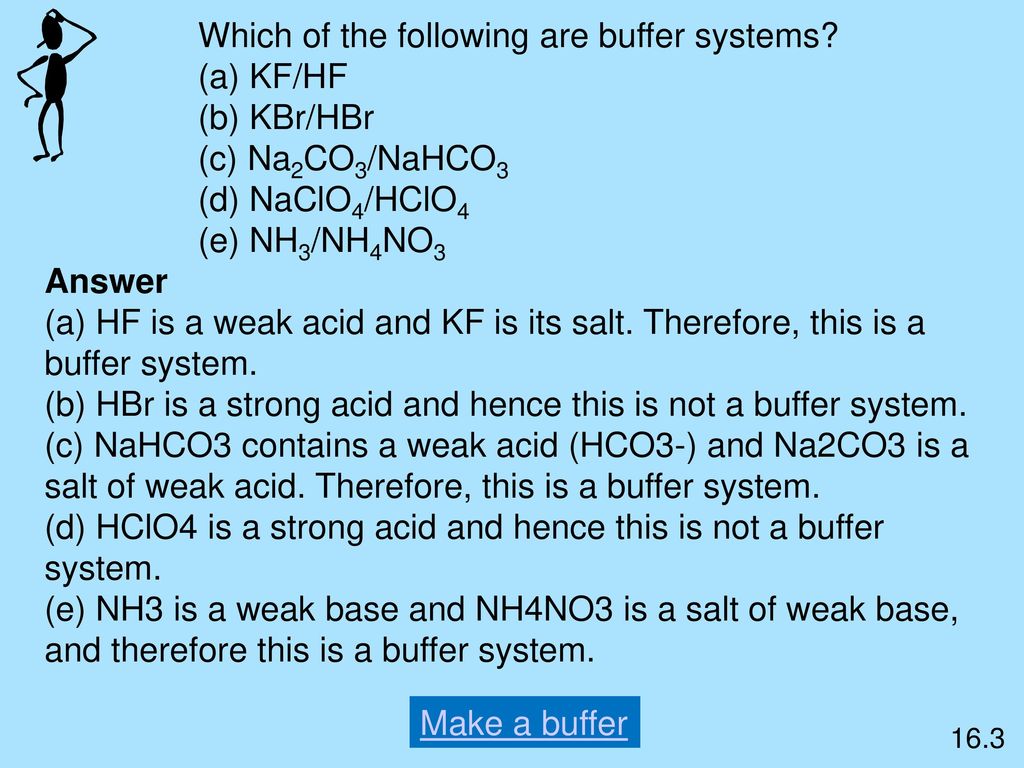
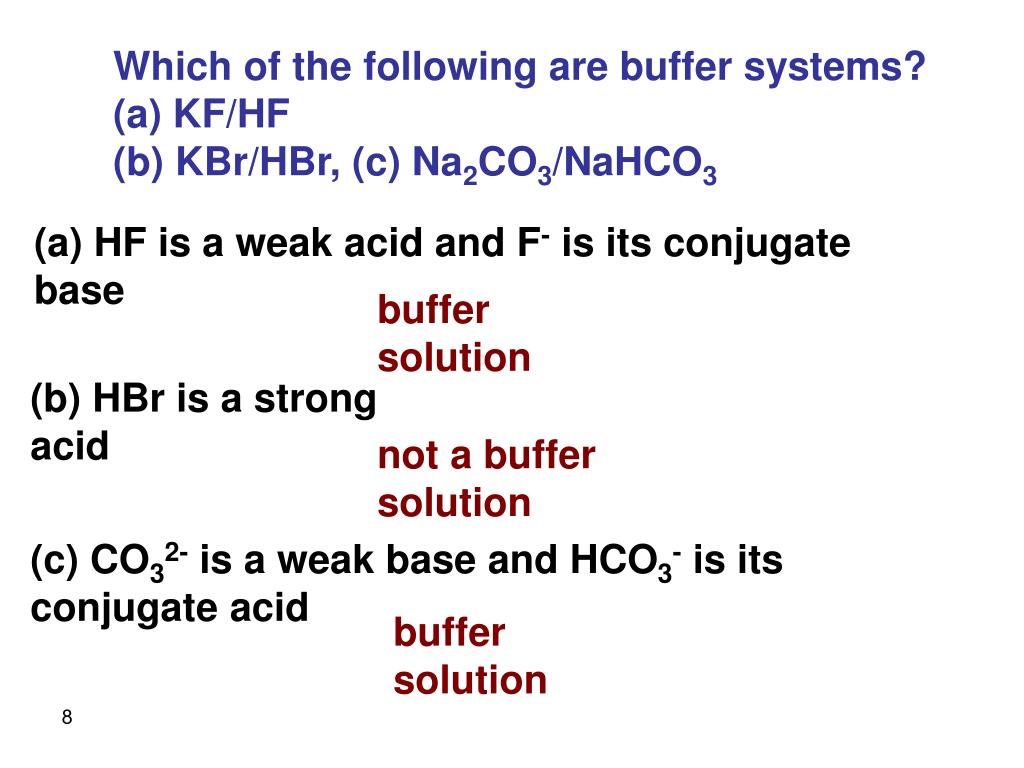
Thus this is a buffer system. H 2 CO 3 and KHCO 3. Its a buffer system that consists of a weak acid and its conjugate base. At the time when the acid is added to it. View the full answer. A protein buffer system B phosphate buffer system C hemoglobin buffer system D NaCl buffer system E bicarbonate buffer system. If acidis added to the solution it is consumed by the conjugate base. A buffer is defined as the mixture of a weak acid with its conjugate base or vice versa. NaOH and NaCl HC2H3O2 and NaC2H3O2 H2S and KHS KCl and NaCl.
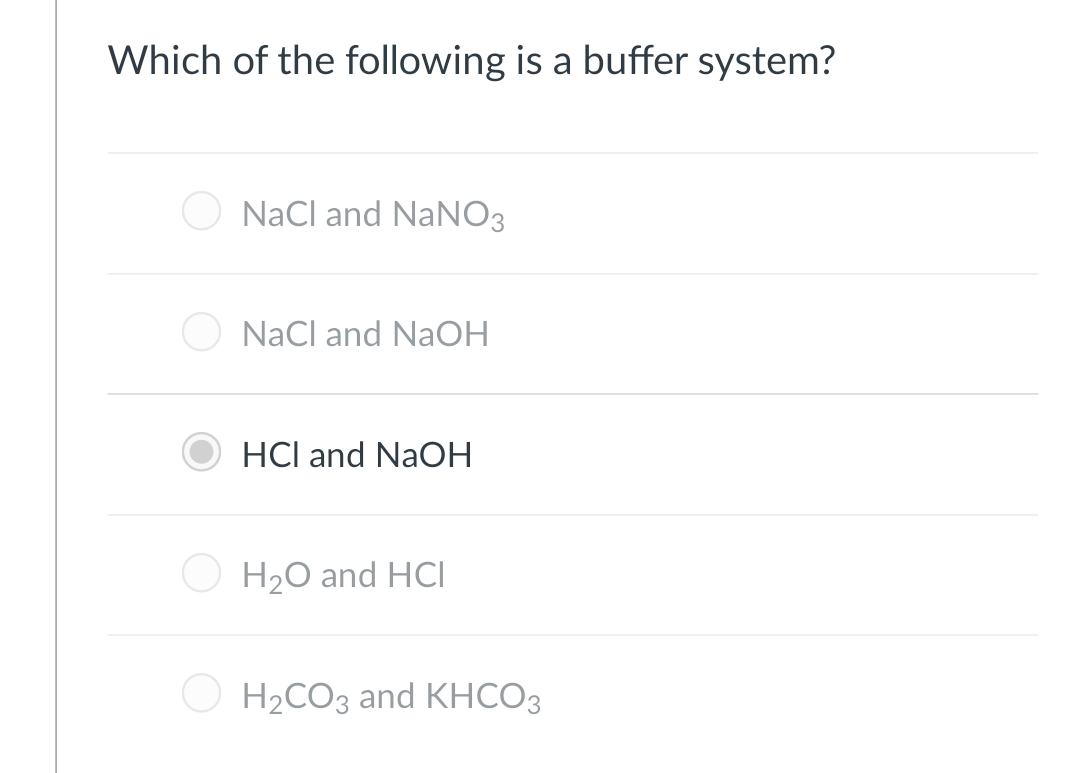
Which of the following is NOT a buffer system of the body. Which of the following is NOT a buffer system of the body. The HCN is a weak acid but its conjugate base is CN that is not present in that solution. More than one correct response O e. Which of the following is a buffer system. HCl and NaOHCH2CO3 and KHCO3DNaCl and NaOH. Storage and buffer management is the liaison to underlying system software and manages retrieval and transmission of data to and from the user and the supported storage mediums including RAM and whatever non-volatile memory is supported by the database.



































Post a Comment for "Which Of The Following Is A Buffer System"